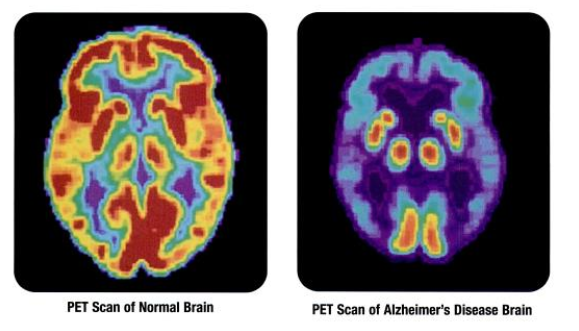
Beyond Covid-19: What Should MedTech Innovators Expect?

Perspectives on Medical Reimbursement By JR Associates In recent months, the coronavirus pandemic (Covid-19) has dramatically disrupted businesses around the world. Arguably, healthcare has been more profoundly affected than any other industry. But even as attention remains focused on virus containment and treatment, medical technology companies must prepare for the long-term. “What next?” is…