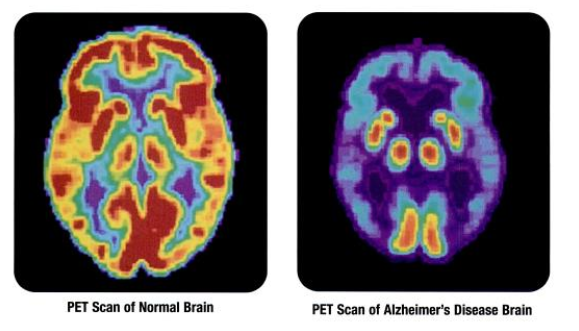
Medical Device Coverage: Do Private Payers Follow Medicare’s Lead?

Perspectives on Medical Reimbursement By JR Associates When making decisions about medical device coverage, do private insurers take their cues from Medicare (CMS)? Not necessarily, according to a recent study published in the peer-review journal, Health Affairs. According to the research abstract, an analysis of CMS national coverage decisions (NCDs) revealed that: ~50% were equivalent to corresponding private payer policies ~25% were…